It is very good for the effects and consequences of technologies to be carefully and thoroughly questioned right from the start and regularly thereafter; environmental aspects are no exception. Mr Sheppard does this in both the first and final sentences of his piece. Sincere debate of merits and flaws is essential for the development of new technology.
For transparency, I'm an engineer who develops commercial hydrogen fuel cell systems professionally for my company, a world leader in commercial fuel cell technology (not UK based). My writing represents my individual views only and is informed by my experience of this topic as we supply fuel cell systems for commercial rail vehicles presently.
My honest view is that the UK would be better off finishing off electrifying its rail network and then supplying it using wind energy. Any hydrogen fuel produced in the UK would be much better used for maritime and long-distance aviation purposes, which remain impractical to conduct without the use of fuel.
This turned into a longer form piece by accident, I appreciate Mr Sheppard did not have as many words to write with as I do - I may well be misinterpreting the intent of his writing at times. I reacted with more detail because it is important to me to represent what hydrogen has to offer in the technical community (as best I am able) and to be open about the risks and drawbacks. Please do question what I write if anything seems unclear, amiss or more sources would be appreciated - many thanks!
___________________________________________________________________________________________________
It was unfair of Mr Sheppard to acknowledge that the HydroFlex project’s vehicle is a demonstrator, then to admonish the practicability of commercial rail vehicles using on hydrogen fuel in general. The deliberate design choices for this demonstrator would make it obviously unsuitable for commercial service, but that doesn’t mean the concept shown would be unsuitable.
An example is Linsinger Maschinenbau G.m.b.H.’s new hydrogen powered MG11 rail milling machine, claimed to be “the only vehicle of its class that fits in the London Underground”. The hydrogen infrastructure is compactly installed above and below the vehicle, leaving plenty of room for the milling equipment: https://www.linsinger.com/portfolio/rail-milling-train-mg11-hydrogen/
To generally question how “green” a technology is in isolation is not an effective way to communicate the present or future quality and value of the technology. My suggestion of a more useful framing is:
For me both hydrogen-fuelled and also directly electrified rail vehicles can tick both of these boxes, making them a “green” and sustainable options for future rail-vehicles.
___________________________________________________________________________________________________
Mr Sheppard describes the CO2 emissions of contemporary hydrogen production correctly. “Grey” hydrogen presently represents the vast majority of global production, where hydrogen is reformed from natural gas. However it is not clearly stated that hydrogen can be produced in large volumes using methods which have no inherent CO2 emissions to the atmosphere, using “blue” or better yet “green” production methods.
I encourage caution regarding “blue” hydrogen: the easiest way to keep the emissions in the ground is to not extract the fossil fuel in the first place. Although a great deal of research and development has been conducted, regrettably a commercial carbon-capture and storage technology is yet to emerge (to the best of my knowledge).
For this reason I am observing projects such as HyNet North West (https://hynet.co.uk/) with great interest, because it is still unclear who is responsible for the CCAS or the capture method chosen.
___________________________________________________________________________________________________
It is responsible to openly acknowledge that molecular hydrogen has inherent properties which mean it can pose a danger, though these also make it useful fuel. Every serious provider of hydrogen fuelled systems makes its safe use the highest priority - accept nothing less from your hydrogen technology partners!
It was unhelpful of Mr Sheppard to describe the hazards of hydrogen vaguely, to then reference two very frightening failures which did not involve hydrogen fuel. Associations of this kind do not help to build confidence for future users of hydrogen technologies. Hydrogen was not a good choice for lighter-than-air flight 92 years ago, as Legh Richardson reminds us, yet this alone does not mean it is an unsafe fuel. I attempt to counteract this statement with the following explanations to offer some reassurance.
Fuels, once synthesised, typically are associated with the following types of hazard:
(1) Hydrogen is not toxic and does not persist in the environment. It being the lightest element in the known universe means it disperses into the atmosphere very quickly and tricky to contain. Even very cold liquid hydrogen disperses very quickly when spilled, as shown in this video from testing conducted in 1960 (61 years ago!) by US Air Force researchers about the flammability of liquid H2: https://youtu.be/7bFJK5kU_UQ?t=146
(2) Compressing (including liquifying) then storing any gas creates a potential hazard should the storage vessel become compromised, irrespective of the flammability of the gas. This is demonstrated in this clip in which a composite type cylinder (similar to those used for storing hydrogen) filled with air to 300 bar experiences a containment failure: https://youtu.be/f-xmaPSZ6GM?t=128
Fortunately industrial gases stored at high pressure in cylinders are used safely every day all over the world, enabling us to make the most of the benefits of storing our gases at high pressure without unacceptably high risk.
Furthermore high pressure containers are inherently much more robust and resistant to external damage than the diesel tanks which fuelled the fire (and severe environmental pollution) in the case of the Llangennech derailment referenced by Mr Sheppard.
(3) Safely using potentially explosive gases is something many of us do daily, for example when using a gas heater, cooker, oven or vehicle with a combustion engine. The specific nature of the risks for hydrogen are well understood. To compare with methane, the majority molecule of compressed natural gas (CNG) and liquid natural gas (LNG) when mixed into atmospheric (STP) air: the minimum flammable mixture “UEG” is very similar for both fuels and a static discharge from clothing would be sufficient to provide the energy to ignite the gas-air mixture of both fuels.
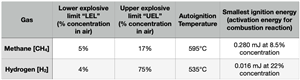
There are more than 10 million CNG-fuelled vehicles in operation around the world, which are generally safely operated despite the inherent hazards. The example of the CNG bus in Stockholm being a rare and upsetting example of it going wrong, though the incident analysis revealed that the explosion should have been preventable.
If the risks can be minimised acceptably for methane, why not with hydrogen?
Source for ignition energy: https://downloadcenter.bgrci.de/resource/downloadcenter/downloads/T033_Gesamtdokument.pdf
Source for LEL/UEL: http://www.wermac.org/safety/safety_what_is_lel_and_uel.html
___________________________________________________________________________________________________
Thank you for your time and reading - have a nice day!
___________________________________________________________________________________________________

IET SEP Editorial:
Last year a hydrogen-powered train travelled on Britain’s rail network with the aim to start carrying passengers by the end of 2021. What are your thoughts on hydrogen technology, key considerations, or risks? Comment below to share your thoughts!
One thing to keep in mind in case of hydrogen fuel cell engine that it gets heated so in case of countries with cold climate hydrogen trains are good but if we consider hot climate regions i.e. countries near equator then it may cause heating problem in engine and gets explode and also the availability of hydrogen should be consistent and storage should be of lower cost for feasibility in greater extent
We're about to take you to the IET registration website. Don't worry though, you'll be sent straight back to the community after completing the registration.
Continue to the IET registration site